How humans (maybe) domesticated themselves
Long before humans domesticated other animals, we may have domesticated ourselves.
Over many generations, some scientists propose, humans selected among themselves for tameness. This process resulted in genetic changes, several recent studies suggest, that have shaped people in ways similar to other domesticated species.
Tameness, says evolutionary biologist and primatologist Richard Wrangham of Harvard University, may boil down to a reduction in reactive aggression — the fly-off-the-handle temperament that makes an animal bare its teeth at the slightest challenge. In this sense, he says, humans are fairly tame. We might show great capacity for premeditated aggression, but we don’t attack every stranger we encounter.
Sometime in the last 200,000 years, humans began weeding out people with an overdose of reactive aggression, Wrangham suggests. Increasingly complex social skills would have allowed early humans to gang up against bullies, he proposes, pointing out that hunter-gatherers today have been known to do the same. Those who got along, got ahead.
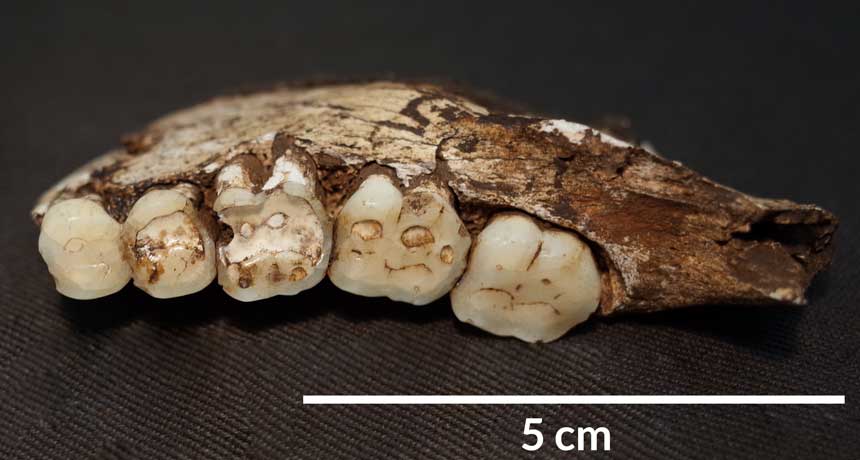
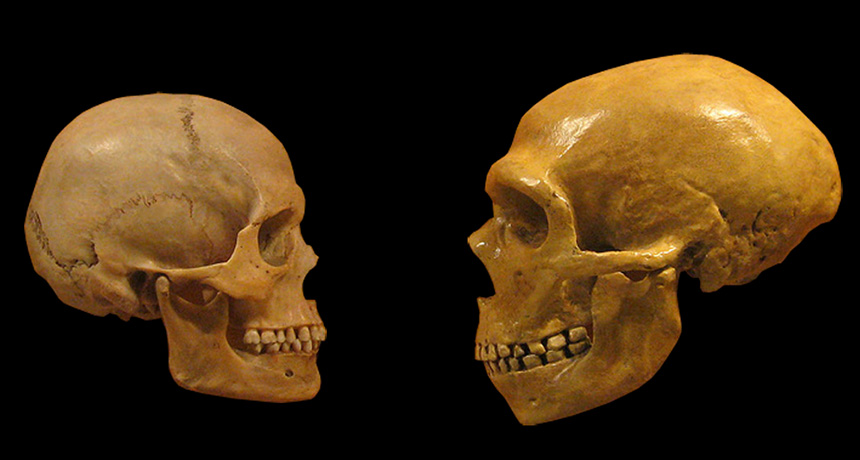
Once animals have been selected for tameness, other traits tend to follow, including reshaping of the head and face. Humans even look domesticated: Compared with Neandertals’, our faces are shorter with smaller brow ridges, and males’ faces are more similar to those of females.
Selecting for less-aggressive humans could have also helped us flourish as a social species, says Antonio Benítez-Burraco, who studies language evolution at the University of Huelva in Spain. The earliest Homo sapiens were becoming capable of complex thought, he proposes, but not yet language. “We were modern in the sense of having modern brains, but we were not modern in behavior.”
Once humans began to self-domesticate, though, changes to neural crest cells could have nudged us toward a highly communicative species. Something similar happens in songbirds: Domesticated birds have more complex songs than their wild counterparts. What’s more, self-domestication may be more common than once thought. Bonobos, Wrangham notes, live in peaceful groups compared with closely related, but more violent, chimpanzees. If humans took steps to domesticate themselves, perhaps they weren’t the only ones.