Gut microbe may challenge textbook on complex cells
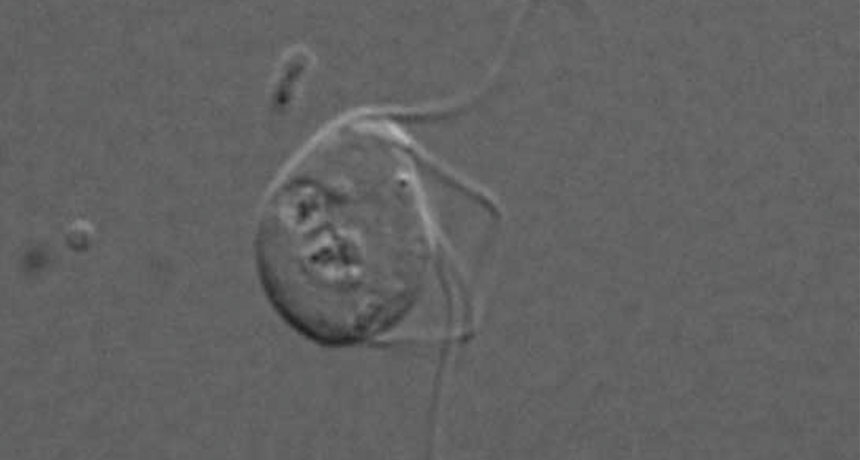
A gut microbe collected from chinchilla droppings might be the first complex life form to lack even a shred of a supposedly universal organelle.
Monocercomonoides, a one-celled gut microbe collected from a pet chinchilla in Prague decades ago, apparently has no mitochondria, the organelles known as the cell’s power plants. Cataloging DNA in the microbe turns up none of the known genes for mitochondrial proteins. But stealing genetic material from bacteria — which survive without mitochondria — allowed the microbe to do without them, too, researchers report May 12 in Current Biology.
Mitochondria are tiny capsules that speckle the insides of all complex cells from pond scum to people, or so textbooks have said for decades. Some complex (or eukaryotic) cells look as if they have no mitochondria; so far, though, further searches have eventually detected mitochondrial remnants.
But Monocercomonoides appears to have completely done away with mitochondria and the genes to make them, says study coauthor Anna Karnkowska, an evolutionary biologist now at the University of British Columbia in Vancouver.
This discovery marks “the most extreme mitochondrial reduction observed,” says Vladimír Hampl of Charles University in Prague, also a coauthor of the study.
The new work also supports the idea that there really is no single core function that defines mitochondria. Although commonly described as cell powerhouses, mitochondria don’t have much to do with supplying energy for cells that live in low-oxygen or no-oxygen environments, Karnkowska says. For these anaerobic cells, mitochondria can serve as more of a building studio. One supposedly essential mitochondrial function, scientists have proposed, is assembling clusters of iron and sulfur that activate a class of widely useful cell compounds.
Bacteria and other simple (prokaryotic) cells have their own assembly systems, and they don’t need to wall off the construction of iron-sulfur clusters. The newly studied Monocercomonoides carry the genes for an assembly system that looks as if it was taken from bacteria, the researchers conclude.
Researchers discovered the lack of mitochondrial genes and the bacterial substitute while working out the DNA components that encode instructions for all the proteins in the whole organism. There were notably no signs of chaperone proteins for conveying other proteins through membranes, something mitochondria do. Nor did other signature mitochondrial proteins show up.
“Pretty amazing story,” says Roland Lill of Philipps University of Marburg in Germany, who studies the way cells use iron. The new paper doesn’t change the basic idea that complex cells need very special conditions, usually created only inside mitochondria, to build their iron-sulfur clusters. “But the beauty of biology,” he says, “is that there are always amazing exceptions to basic biological rules.”